Precision in Assembly: The Key to Excellence for Manufacturing Companies
9 Ways Clear and Unambiguous Manufacturing Assembly Work Instructions Benefit Companies that Produce Complex Products like Medical Devices
The Foundation: Accurate & Clear Manufacturing Assembly Instructions
Manufacturing engineers play a pivotal role in creating clear and precise manufacturing assembly instructions – and these instructions must be unambiguous. For medical device manufacturing, their role is foundational to a smooth and efficient assembly process. The assembly planning process starts with the complete set of all 3D CAD files from the design department.
9 Benefits Visual Work Instructions Bring to the Medical Device Manufacturing Company
 Consistency in Operations: Clear assembly instructions ensure that all assembly line workers follow the same set of guidelines, promoting consistency in manufacturing processes. For example, precise instructions for the installation of intricate medical devices, such as pacemakers or infusion pumps, guarantee uniformity in production.
Consistency in Operations: Clear assembly instructions ensure that all assembly line workers follow the same set of guidelines, promoting consistency in manufacturing processes. For example, precise instructions for the installation of intricate medical devices, such as pacemakers or infusion pumps, guarantee uniformity in production.- Quality Control:
 Unambiguous assembly instructions enable better control over the quality of medical devices. Clear assembly instructions for critical components, like sterilized catheters or implantable sensors, ensure high-quality and reliable performance in every device produced.
Unambiguous assembly instructions enable better control over the quality of medical devices. Clear assembly instructions for critical components, like sterilized catheters or implantable sensors, ensure high-quality and reliable performance in every device produced.  Reduced Errors and Rework: Ambiguities in assembly directions can lead to mistakes and errors, causing a need for rework. Utilizing a well-defined assembly instruction template minimizes these errors. In medical device manufacturing, explicit guidelines for the assembly of drug delivery systems can prevent defects and subsequent production-line rework.
Reduced Errors and Rework: Ambiguities in assembly directions can lead to mistakes and errors, causing a need for rework. Utilizing a well-defined assembly instruction template minimizes these errors. In medical device manufacturing, explicit guidelines for the assembly of drug delivery systems can prevent defects and subsequent production-line rework. Efficiency Improvement: Well-defined assembly instructions streamline the medical device assembly process, making it more efficient. Work instructions for assembly, covering components like micro-electronics and precision optics, can significantly reduce production time and increase overall assembly plant efficiency.
Efficiency Improvement: Well-defined assembly instructions streamline the medical device assembly process, making it more efficient. Work instructions for assembly, covering components like micro-electronics and precision optics, can significantly reduce production time and increase overall assembly plant efficiency. Training and Onboarding: Clear assembly instructions facilitate the training of new workers and streamline the onboarding process. For example, detailed assembly work instruction templates support fast adaptation within the specialized medical device manufacturing environment for complex systems – including surgical robots or imaging equipment.
Training and Onboarding: Clear assembly instructions facilitate the training of new workers and streamline the onboarding process. For example, detailed assembly work instruction templates support fast adaptation within the specialized medical device manufacturing environment for complex systems – including surgical robots or imaging equipment. Standardization: Unambiguous work instructions contribute to standardizing medical device assembly processes. Production of widely used medical devices relies on standardized methods – from sterile packaging to quality control testing – guided by instructions to meet safety standards and regulatory requirements.
Standardization: Unambiguous work instructions contribute to standardizing medical device assembly processes. Production of widely used medical devices relies on standardized methods – from sterile packaging to quality control testing – guided by instructions to meet safety standards and regulatory requirements. Risk Mitigation: Ambiguous assembly instructions can lead to patient safety hazards. Clear and precise instructions reduce the likelihood of failures or malfunctions throughout the assembly process. In medical device manufacturing, well-defined instructions with calibration processes and testing protocols reduce operational risks and contribute to patient well-being.
Risk Mitigation: Ambiguous assembly instructions can lead to patient safety hazards. Clear and precise instructions reduce the likelihood of failures or malfunctions throughout the assembly process. In medical device manufacturing, well-defined instructions with calibration processes and testing protocols reduce operational risks and contribute to patient well-being. Communication and Collaboration: Clear assembly line instructions facilitate effective communication within medical device manufacturing. Collaborative efforts improve when instructions provide detailed insights into design intent, improving the overall quality and performance of the device.
Communication and Collaboration: Clear assembly line instructions facilitate effective communication within medical device manufacturing. Collaborative efforts improve when instructions provide detailed insights into design intent, improving the overall quality and performance of the device. Regulatory Compliance: In medical device manufacturing, compliance with FDA regulations is critical. Manufacturing instructions help ensure strict adherence to industry regulations. Detailed protocols for handling biocompatible materials and sterilized components contribute to compliance with healthcare safety standards.
Regulatory Compliance: In medical device manufacturing, compliance with FDA regulations is critical. Manufacturing instructions help ensure strict adherence to industry regulations. Detailed protocols for handling biocompatible materials and sterilized components contribute to compliance with healthcare safety standards.
How Can XVL Help?
3D CAD is great for the design department, but not for the rest of the company.
XVL takes off from where your 3D CAD leaves off.
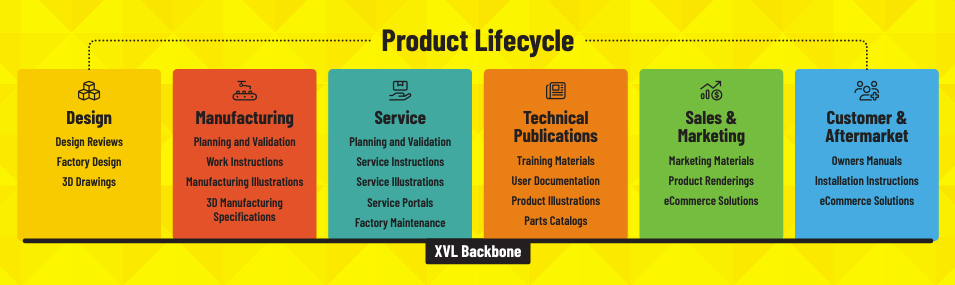
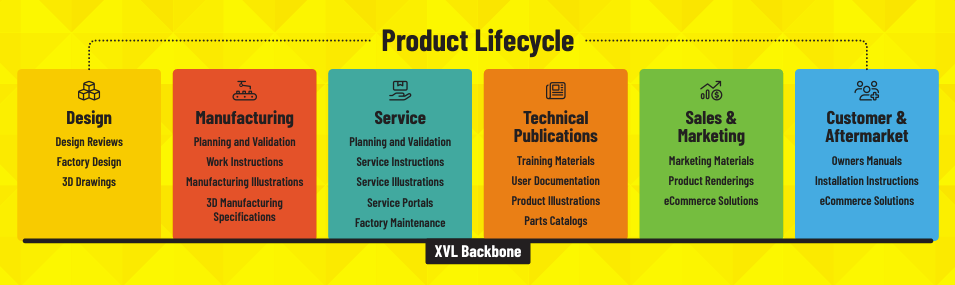
Complete Virtual Product to Support the Complete Product Life Cycle
With XVL, any stakeholder throughout the product lifecycle can access the complete virtual product with intuitive and easy access. You probably have a PLM or PDM system that contains the design data, but it is easy and intuitive for stakeholders to access.
XVL Provides Complete Data and Fast Performance
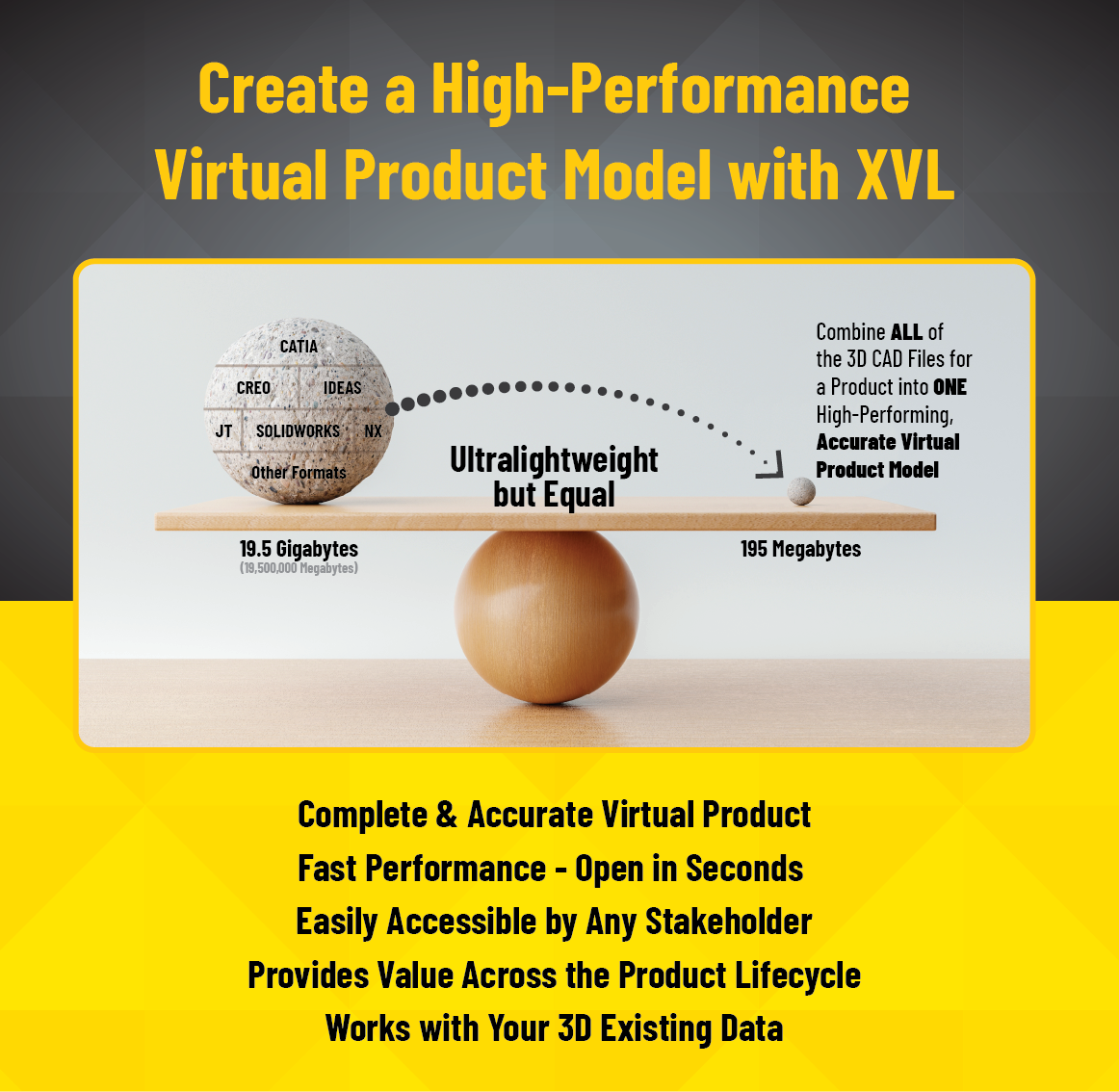
With XVL, all 3D CAD data (even different formats) is combined into a SINGLE Virtual Product Model. XVL retains CAD-level precision and all metadata from the originating CAD system.
And with XVL, function-specific information can be added (for example assembly work instructions and service instructions).
And data can be pulled from XVL for example to create technical illustrations.
Summary
Having an efficient product assembly process is crucial for the medical device manufacturing company. It affects everything from time-to-market to operational profitability, to customer satisfaction.
In summary, manufacturing engineers are indispensable in creating clear and precise work instructions, based on thorough assembly process planning. Complex medical devices may contain hundreds or thousands of parts which creates challenges for manufacturing planning.
XVL can help by enabling the creation of a single Virtual Product Model that all stakeholders can easily access and use for their roles. Submit a question or your challenge below and one of our solution architects will get back to you.